Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
An Overview and Perspectives of Nanoparticles Against Cancer Treatment
*Corresponding author:Kainat Ramzan, Saira Ramzan, Department of Biochemistry, Department of Zoology, Faculty of Life sciences University of Okara, Okara-56130, Pakistan.
Received:January 02, 2023; Published:January 19, 2023
DOI: 10.34297/AJBSR.2023.17.002410
Abstract
In past decades, the evolution of nanoparticles has tried to expand into a broad range of clinical purposes. Nanoparticles (NPs) bring tremendous potential for enhancing disease diagnosis and therapeutic selectivity. Due to their unique properties, NPs have emerged as a promising technique for treating cancer. As organic, polymeric, and inorganic NPs become more precisely formed, they can be improved for more customized drug carriers. Nanotherapeutics is now a rapidly growing area of cancer research that is being utilized to resolve a few drawbacks of drug delivery. Daniel Chen and Ira Mellman defined the cancer-immunity cycle, as a set of self- sustaining sequential events wherein anti-cancer immune cells lead to the efficient eradication of tumor cells. Passive targeting enables nanocarriers to collect in cancerous cells via the EPR effect, whilst active targeting is accomplished by conjugating NPs to over-expressed antigens or receptors on cancer cells. Nanoparticles have distinct physiochemical properties and prepared using physical, biological, and chemical processes that are used to fight cancer by provoking, improving, or suppressing immune reactions. Tumor therapies utilizing the nano-platform remain in the pre-clinical stage, and NPs themself elicit immunomodulatory cell apoptosis and evoke both native and adaptive immune responses for proving a widespread anti-cancer process.
Keywords: Nanoparticles, Cancer Treatment, Histocompatibility Complex
Introduction
According to Global Cancer Statistics Report, nearly 19.3 cases of cancer and 10 million deaths were reported in 2020. Incidence of cancer has now been rising over the years due to a variety of causes like as demographic growth, work pressure, and ecological factors [1]. Nanomedicine has been thoroughly explored and used in cancer treatment because nanoparticles (NPs) can serve as an effective drug delivery mechanism. Micro based NPs drug delivery has distinct benefits over traditional drug carrier, including increased stability and bioactivity, increased permeability and retention (EPR), and particular target specificity [2]. Over the last decade, approximately 12,000 studies on nano-based carriers in cancer treatment were reported. Multiple NPs drugs have been produced,Liposomes were discovered in 1965 by a group led by Bangham [3,4]. In 1995, the US Food & Drug Administration (FDA) approved doxorubicin liposomal formulation (Doxil) for the treatment of AIDS-related Kaposi sarcoma. In 2005, the FDA approved protein-bound paclitaxel (Abraxane), an albumin-based nanoparticle, for breast cancer treatment, non-small cell lung cancer, and pancreatic cancer [5].
In 2013, ado-trastuzumab emtansine (Kadcyla) drug became approved to treat patients with human epidermal growth factor receptor 2-positive breast cancer [1,6,7]. Immunotherapy was not the only therapeutic application of NPs, the authors of Science declared immunotherapy the “Breakthrough of the Year” [8]. For many years, cancer treatments were restricted to surgical, radiotherapy, and chemotherapy. These 3 approaches pose a risk to normal tissues or lead to inadequate cancer elucidation. Traditional chemotherapy suffers from the lack of drug dissolution rate, a failure of specific tumor cells, and multidrug resistance created by chronic exposure to the same treatment [9,10]. The emergence of drug-resistant exhibits a difficulty in anticancer therapy and overcome by utilizing polymeric NPs, mesoporous silica NPs, chemosensitizers, nanoemulsions and nanocrystals [11].
Additional therapies that are beneficial for antitumor immune responses [12,13]. Our immune cell shows a substantial innate ability to recognize and kill aberrant cells. New treatments can boost the activity of immune defense components or oppose signals generated by cancerous cells that decrease immunological responses [14,15]. In general, our immune system defends us from cancer by performing three major functions: 1) Trying to eliminate virusinduced cancer infestation, 2) Resolving inflammatory response that promotes tumor progression, and 3) Identifying cancer cells relying on their interpretation of tumor-specific antigens [16]. The immune system triggers 2 kinds of immunological responses: innate and adaptive [17]. Immunotherapy could be used to either inhibit or boost immunity, based on the particular outcome. The most frequent characterization of therapy is the contrast between active and passive therapy [18,19]. Active immunotherapy involves priming the immunity to respond to antigens, whereas passive immunotherapy involves inducing an innate immunological role through the administration of immune-stimulating substances [20,21].
Under this context, we investigate whether NPs can go beyond merely offering therapeutic distribution routes, as well as how unique NPs can be engineered to have intrinsic immunomodulatory features that contribute to increasing anticancer therapy. Such NPs provide a promising opportunity to advance methods that might someday greatly improve tumor treatment, whether the development of a more efficient immunomodulating delivery agent or the introduction of advanced nanomaterials that can preferentially govern immunologic cellular activities. A few of the significant immunotherapy approaches are given below.
Immune Checkpoint Modulators
These are antibodies that typically regulate immune function by inhibiting overly aggressive reactions. Immune checkpoint protein regulates immunogenicity and improves its potential to lessen tumor cells [10]. In 2018, James Allison and Tasulu Honjo identified immunological checkpoints, and have been granted the Nobel Prize in Medicine or Physiology [22]. Antibiotics against Cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death-1 (PD-1), and programmed cell death ligand-1 (PD- L1) are used in an immune checkpoint, and PD-1 inhibitors were approved by FDA for cancer and Hodgkin’s lymphoma [23,24].
Oncolytic Viruses
Oncolytic viruses are a diverse group of DNA/RNA viruses that are inherently tumor-specific and bioengineered. Firstly, a virus is inserted into the cancer and then targets the cancer cells to repeat itself. As cells erupt or died by releasing a specific material known as antigen and activate the immunogenicity. In 2015, FDA initiated an oncolytic viral platform to treat melanoma [25]. Such OVmediated viruses are divided into two types: those that proliferate in tumor cells and those that are non-specific in humans due to a greater susceptibility to antiviral signals. For instance, these are independent parvoviruses, myxoma virus, para-myxo virus, and reovirus. Some genetically modified vectors are the measles virus, poliovirus, and vaccinia virus [26]. A fundamental goal of OV-mediated therapies is the activation and targeting of effective immune effector cells against tumors [27-29].
Chimeric Antigen Receptor T-Cell therapy (CAR-T Cell Therapy)
CAR-T cell therapy, a more refined type of Adoptive Cell Transfer (ACT) method that takes T cells from a donor, has been explored for the therapy of certain cancer types and infections [30]. T cells are modified to produce after collection to make chimeric antigen receptors [31]. These modified CAR-T cells are controlled by the lab until they number within billions and the donor is then given the increased population of CAR T- cells. Such T cells then grow in the patient’s body, identifying and attacking tumor cells with the help of their receptor [32]. Despite recent advances in genomics, there is growing interest in using NPs to transport siRNA for transcribed regulation or cas9 mRNA to recover disease-associated genes in vivo. Synthetic DNA was able to convert circulating T cells into an anticancer profile by inserting leukemia-targeting CAR genes into the nucleus of such T cells [33,34].
Antibody-drug Conjugates (ADCs)
Therapeutic antibodies, known as ADCs, are created in the labs to attack tumor cells and shown to be highly potent in inducing apoptosis. The ADCs antibody module enables it to engage with a target molecule on the cancer cell’s surface [35]. When an ADC adheres to a tumor site, it absorbs the cell’s poisonous contents and destroys the cell. Vaccines are generally made from a patient’s cancer cells or chemicals and compounds produced by tumors. They were intended to cure pre-existing cancers by enhancing the body’s immune strategy against cancer [36,37].
Monoclonal Antibodies (mAbs)
Various mAbs are useful for cancer treatment as they have a particular target on tumor cells. Some antibodies act similarly to immunotherapy including that they strengthen the immune response, allowing the body to detect and fight cancerous cells [38]. The mAbs offer a variety of forms, naked mAbs are free of any medication or radioactive substance and are prevalent mAbs used for cancer therapy. The first medicine Rituxan (rituximab) was approved by the FDA for non-lymphoma Hodgkin treatment [39]. Functionalized mAbs were labeled and injected with a chemotherapeutic or a radioactive particle. These serve as a homing mechanism for delivering a specific drug exactly to cancer cells [40]. The mAb flows across the body until it encounters the specific antigen. While distinct mAbs are formed by components of two separate mAbs, they may bind to two distinct proteins at the same instance. For instance, Blinatumo mAbs is utilized to treat certain forms of leukemia [41,42].
Effect of Nanomedicine Towards Immune Response
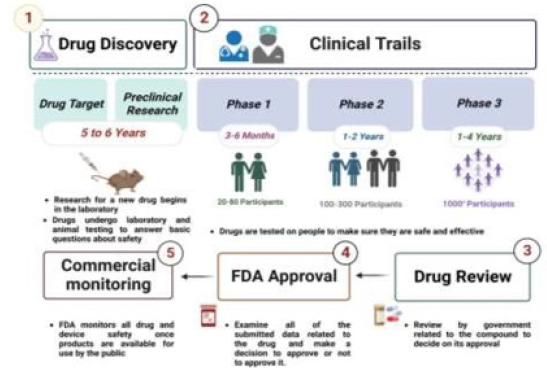
The FDA and the European Medicines Agency (EMA) have both approved a range of NP-based medicines for tumor therapeutic and diagnostic uses, and many other formulations are now being assessed [43,44]. Nearly 250 products were approved for sale or a variety of clinical phases for investigation [45]. The FDA divides drug development and approval into three primary stages, as shown in Figure 1. After the identification, the pre-clinical testing phase often consists of animal experiments to demonstrate activity, stability, and toxic levels, and also to identify suitable dosage limits [46,47]. Because the FDA approval procedure is time-consuming, labor-intensive, and competitive, it is believed that creating a new therapy takes roughly 10-15 years [48].
Moreover, the developing field in immunogenicity is the discovery or design of NPs which can control individual phases of the immunomodulating process. For instance, Ferumoxytol, an iron oxide NPs affirmed by the US FDA for treating anemia, has lately been shown to evoke the charge separation of tumor-associated macrophages and encourage superoxide radicals (ROS) mediates the tumor cell death [49]. The NPs surfaces can be modified to improve cancer cell ingestion and antigen presentation by macrophages. To use a bispecific multifarious nanocarrier design, receptortargeted HER2-positive cells in breast cancer by phagocytosis can be eliminated via pro-phagocytic signaling regulated by calreticulin [50]. Because of their great physiochemical properties, low toxicity, strong biocompatibility, and the ability to load adjuvants and antigens simultaneously, NPs are ideal candidates as a platform for innovating cancer nano-vaccines [51,52].
Also, NPs usually exhibit colloidal stability issues, such as low dissolution rate in the bloodstream and adverse interactions with reticuloendothelial system cells like macrophages [53]. In 2018, James P. Allison and Tasuku Honjo have received the Nobel Prize in Physiology or Medicine for their research in cancer immunotherapy [54,55]. Following this discovery, NPs began to be employed as immunotherapeutic agents, and certain antitumor medicines relying on NP compositions were approved by FDA. Moreover, such systems encounter challenges with physiological medium stability, PC formation by biofluids, and deposition inside the tumor site [56]. The primary role is modifying the physiochemical characterization of the nanocomposites, leading to a decrease in the therapeutic effects of nanomedicines [56,57].
Nanocarriers
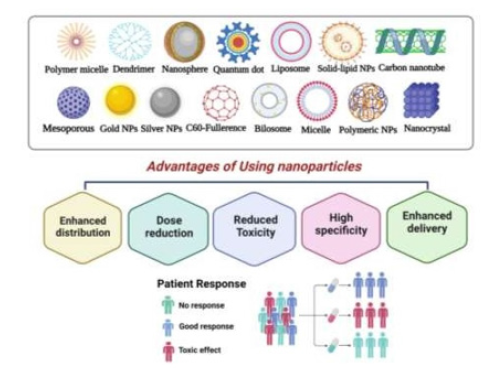
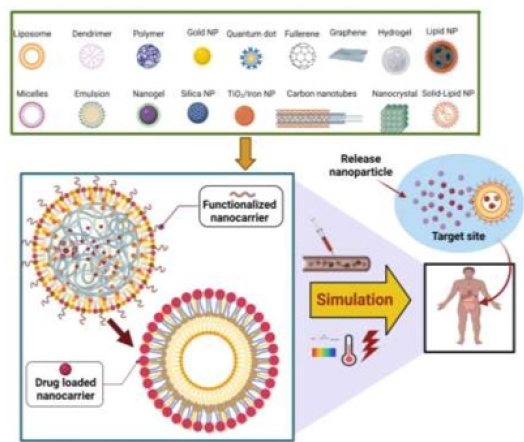
The basic concerns in cancer therapy are NPs utilized as carriers to rapidly transfer drugs into tumor tissue while avoiding noninfected cells. The size of the NPs should be tiny on the nanoscale, to pass over diverse biological and physiological obstacles [58]. Most drugs rarely flow through blood vessels with diameters of 5-6 microns. Such NPs compounds require a diameter (10-100 nm) to access various body parts [59,60]. Having a high surface-to-volume ratio, they have a fast dissolving ratio while poorly soluble NPs get a significant dissolution rate, for instance, Paclitaxel, Amphotericin B, or Cyclosporine [61]. The particle charge, surface morphology, and hydrophobicity of NPs reduce the undesirable effects. Various nanocarriers can be created by lipids, polymerics, proteins, metals, and semiconductors material [62]. A particle can be circular, tube- shaped, or rod-shaped, and various shapes were proposed. As shown in Figure 2, NPs are classified into three types: organicbased, inorganic, and polymeric nature. However, NPs delivery systems can protect pharmaceuticals from decay as well as boost cancer therapy, inhibit drugs from engaging with healthy cells and avoid adverse effects [63,64].
The Cancer-Immunity Cycle
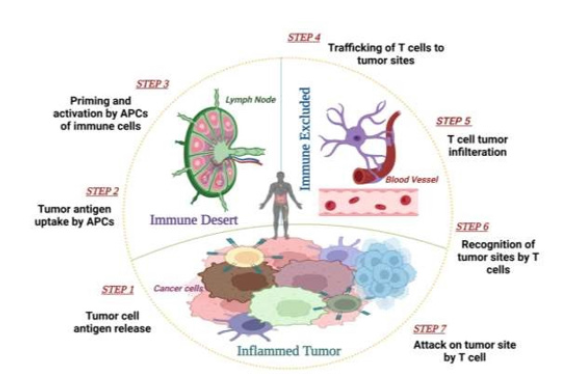
The association of cancer or lymphatic cells assists in the creation of effective treatments that stimulate the immune reaction [65-67]. Immunotherapy attempts to recruit human defense systems in T-lymphocytes and also cytotoxic cells in the tumor microenvironment, with aim of making it a conventional therapy for cancer victims [22]. NPs were examined for their ability to rapidly distribute drugs, defend against enzymatic degradation, and persist in the bloodstream for long durations. We first reviewed the concept of antitumor therapy before examining the usage of NPs to target cancerous cells [68]. In 2013, Daniel Chen and Ira Mellman identified the cancer immunity cycle as a set of sequential events that result in an effect of tumor cells by anti-cancer immune reaction [67,69]. T-cell immunity improved tumor immunotherapy by using the basic steps: Cancer antigens must be transmitted to lymphocytes, especially antigen-presenting cells (APCs). Cancer antigens stimulate T cell priming by the filtering of activated tumor- specific cytotoxic T cell receptors (TCLs). Such TCLs engage with T cells to cause cell apoptosis that induces cancer antigens elimination, which improves the immune response [23,63,70].
Mechanism of Cancer Immunotherapy
The tumor cells induce necrosis or apoptosis by yielding cancer antigens that are taken by APCs to generate peptides that bind to the major histocompatibility complex (MHC). Certain of these antigens, referred to as Neo-antigens, are tumor-specific and not present in healthy cells [63,67], peptides bind to MHC-I and MHC-II molecules and are presented to T lymphocytes. MHC-II peptide can be recognized by the CD4+ T cell receptor. Dendritic cells carrying cancer antigens migrate to lymph nodes, where they excite immature T cells and activate TCLs, which detect tumor cells by engaging with T- cell and MHC complex. Activated T cells migrate to the tumor site, infiltrate it, and bind to cancer cells. Lastly, T lymphocytes kill cancer cells by initiating a sequence of events that trigger cell damage by apoptosis, allowing the cycle and anticancer response to persist [71,72]. As illustrated in Figure 3, these processes all play a significant role in inducing effective anticancer immunity [69].
Moreover, tumor antigens are produced by necrosis or apoptosis, which strengthens the immune reaction, which is unfortunately delayed by many obstacles. When pro-inflammatory cells, such as M1-polarized macrophages, destroy tumor cells, and produce immunosuppressive substances such as IL-10, TGFbeta, and sphingosine-1- phosphate (S1P), prompting phagocytes to repolarize [73]. The apoptotic cancer cells secrete monocyte chemo-attractant protein-1 (MCP-1) induces monocyte infiltration into the tumor microenvironment. Such monocytes develop into tumor-associated macrophages (TAMs), that promote cancer progression and allow cancer to elude immune control. Myeloidderived suppressor cells release anti-inflammatory cytokines, activating immunosuppressive regulatory T-cells (Treg), which limit dendritic cell maturation. As a result, pharmacological treatments or nanomaterials are used in many phases to enhance anti-cancer immunity [63,67].
Antigen-presenting cells identify tumor antigens produced by tumor cells. Matured APCs travel to lymph nodes, where they prime and multiply T lymphocytes. T lymphocytes that were activated by APCs are directed to cancerous tissue and induce apoptosis. Cancer antigens derived from dying cancer cells trigger yet another immune reaction, leading to a cancer-immunity cascade.
Cancer Antigen Delivery Through Nanoparticles
Tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) are two types of tumor-associated antigens. TAAs are antigens that appear more frequently in cancer cells or at the differentiation stage of cancer cells [76]. While neoantigens, also known as TSAs, are only found in cancer cells, these native tumor antigens are rapidly destroyed by enzymes in the body and have low sensitivity [77]. As a result, NPs were extensively examined for delivering specific antigens to lymph glands. Nanotechnology offers two significant benefits under this overview: it can preserve tumor antigens from degradative enzymes and also enable selective delivery to lymph glands. The distribution of nanomaterials to lymph is strongly dependent on the size of particles, charge density, form, and surface chemistry. Small particles can escape from blood vessels throughout the circulation, but large particles might become stuck in the ECM and are limited to lymph nodes [74,78].
Furthermore, medium-sized nanoparticles circulate effectively and are fed to lymph glands. It was found that polypropylene sulfide particles were ingested into lymphatic arteries and delivered to lymph nodes for up to 120 hours, but only half of such particles were found in the lymph node, DCs, and APCs. The 25 nm NP is efficiently delivered to flow out lymph vessels via the tissue fluid and the following intra-dermal injection, whereas only 10% of the NPs were delivered directly [79]. As a result, NPs having 5-100 nm efficiently transfer tumor antigens to lymph nodes. While most are spherical, recent breakthroughs in nanotechnology have exploited a range of different shapes such as rods, pyramids, squares, stars, and discs. Figure 4 depicts how well the carrier’s surface affects cellular uptake and immune reaction stimulation [80,81].
In general, positive charge particles perform a more significant effect on immune reaction than negatively charged particles. Because positive charges are frequently fixed in negatively charged ECM, they possess lower cell permeability. If specific dendritic cells are confined at the injection site, they are more likely to absorb positive particles than opposite charges [82,83]. The positive charge density might trigger hemolysis and platelet aggregation, resulting in immature antigen release. A recent study has shown that polymers with hydrophobic domains, such as PLGA and chitosan, might activate immune cells. For example, amphiphilic NP with poly gamma-glutamic acid (PGA) improves antigen absorption and activates the DCs, as well as cell growth [74].
Drug Delivery Targeting
Targeting refers to NPs ability to reach cancer sites selectively with low therapeutic loss in the bloodstream. Generally, two major categories were proven to increase patient survival by improving internalized drug levels and avoiding toxic effects. Figure 5 shows how NPs deliver in either active or passive targeted modes [84,85]. Passive targeting permits NPs to aggregate in a tumor by EPR effect, whilst active targeting is performed by mixing NPs that adhere to many antigens or receptors on cancer cells. Various nanoformulations of antigenic, chemokine, inflammatory cytokines, nucleotides, and Toll-like receptors target diverse lymphocytes have been effectively demonstrated and yield positive outcomes. Therefore, nanomedicine primarily functions as a medium that allows for more effective and specific delivery of immunomodulatory drugs to aid in the activation of anticancer immune function [86,87].
DPassive Targeting
Passive targeting is the accumulation of drugs at a specific area that allows the NPs to remain in the tumor site and manifests as an inflammatory process in a certain area. Rapidly growing cancer recruits new capillaries to hypoxia, allowing selective improved penetration of macromolecules to the tumor microenvironment [88,89]. Tumor vessels are extremely disordered and have gap junctions (holes) among endothelial cells. The vascular barrier is damaged at tumor sites, allowing nanocarriers to aggregate in tumor tissue. Due to poor lymphoid activity, NPs are not immediately removed and collected in the tumor tissues. This is known as the enhanced permeability and retention (EPR) effect, and it serves as the foundation for passive targeting [41,90-92]. Nanoparticle deposition is determined largely by physicochemical variables like size, structure, charge density, and surface composition [22,93]. Tumors have poor lymph drainage and leaky blood vessels, which allow NPs to aggregate in cells. Malignant cells and inflamed cells are a few disorders where passive targeting can be performed [23,68].
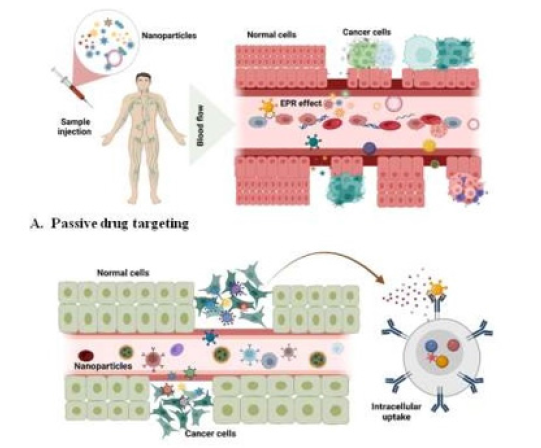
Figure 5:Schematic illustration of active and passive drug targeting of nanoparticles. A) Passive tissue and B) Active cellular tissue targeting are the two forms of targeting that nanoparticles can initiate. To avoid the buildup of nanoparticles in unexpected sites, active targeting is preferred over passive targeting.
In 1986, Matsumara and Maeda were the first to discover the EPR effect [84]. Encapsulating molecules improves systemic circulation, promotes cell specificity, and lowers adverse effects. This sort of targeted delivery does not have cell and tissue interaction. Liposomes that are more sophisticated are bound with a synthetic polymer that protects the drug from immunological degradation [94]. Increased levels of vascular mediators could explain the poor and defective tumorigenesis such as nitric oxide, endothelial, and fibroblast growth factors. Furthermore, the milieu around tumor cells differs from healthy tissue, fast-growing cancer cells demand more nutrients and oxygen because of their high metabolism. As a result, glycolysis serves to generate extra power in an acidic medium [95]. Liposomes are pH sensitive and are stable at pH 7.4 [96]. Cancer cells also secrete enzymes involved in their mobility and maintenance, such as matrix metalloproteinases. Although the passive target approach has some drawbacks [97]. Several drugs do not disperse effectively, and the randomness of the method makes it difficult. The passive method is further constrained since these cancers may not exhibit EPR effects and vascular permeability [85,88].
Ligand-mediated Targeting (Active Targeting)
Ligand-mediated targeting is focused on the particles present on the surface of cancer cells and selective targeting improves the drug effects by attaching a molecule to the NPs that enables targeting of the molecules to express a precise binding site. The evidence of active targeting was proposed in 1980 using antibodies placed on the surface of liposomes, followed by different types of ligands such as peptides, nucleic acids, and epitopes. Also, the study explains a case in which CNS and glioblastoma were treated by targeting nicotine acetylcholine with micelles [88]. The active targeting concept is utilized to transport NPs into cancer, which recognizes and binds to target cells via the expression of receptors or epitopes on the cell surface [23]. Such agonists have a high selectivity for receptor and other cancer-specific sites which are highly expressed on the surface of cancer cells. Transferrin, folic acid, enzymes, modified antibodies, and polymers are a few types of ligands. The density should be adjusted so that NPs do not become recognized by the reticuloendothelial system or interact with serum proteins, hence extending their diffusion rate [90]. Moreover, the ligands must be expressed uniformly and not leaked into the bloodstream. Endocytosis of targeting conjugates can also occur via receptor-mediated endosomes. Both active and passive targeting are used in tandem to reduce therapeutic conflict and promote drug penetration by improving the NPs binding with the surface of the cancerous cells [88,89,91,93,98].
Physicochemical Features for Effective Cancer Immunotherapy
Nanoparticles made from various materials can encapsulate antitumor agents inside their interior cores to improve specific drug aggregation within a cancerous cell [99]. Such inherent qualities of NPs have led to their use in immuno-oncology purposes. Higher reactivity, compact size, and a larger surface area are physicochemical factors that influence drug efficiency. These properties can be used to address some of the flaws of modern medical and screening drugs [100]. For nanomaterials, certain variables such as charge density, size distribution, structure, solubility, surface, crystallinity, consistency, surface energy, and are highly crucial [101].
Size
The average size of NPs governs numerous features including intracellular delivery, tumor aggregation, rate of diffusion, modes of action, antitumor activity, and toxic effects [102]. The size of NPs can range from 1 to 100 nanometers, with the maximum size exceeding 1 micrometer [57]. Small nanoparticles (39 nm) are readily absorbed by pancreatic cancer cells and showed better activity [103]. A small particle size leads to an overall increase in surface area, particles with a similar content could have significant differences in medicinal and lethal potency [104]. A study was conducted to determine variations in tumor mass influenced cancer deposition and absorption of different size globular gold nanoparticles (AuNPs). It became revealed that deviations in cancer cell etiology related to tumor size can result in specific alterations in cancer absorption of differently sized NPs [105]. The 20-200 nm of NPs is commonly given up via endosomes, whereas particles more than 500 nm are typically overtaken via phagocytosis. As a result, particle size plays a vital role in modulating vaccination activities [106].
Shape
Nanoparticles are classified according to their shape, such as nanospheres, nanocapsules, nanocages, nanotubes, nanorods, nanoribbons, nanosheets, and so on [107]. One study explored the photo-thermal cytotoxic effects of functionalized iron oxide (Fe3O4) of varying forms such as circular, hexagons, and cables. The Fe3O4 nanoparticles demonstrate strong photodynamic effects when exposed to red and near-infrared laser beams [108]. The delivery method to directly attack its intended sites and used to improve vascular permeability due to the EPR effect. The physical and chemical features of NPs determine the effectiveness of the EPR effect [109]. In vitro assay, the effect of particle shape was already found to be crucial in cellular uptake. Uptake of the disc-shaped particle is favored in immunological and epithelial tissue owing to its high ratio, as opposed to low aspect nanotubes [110].
Surface Chemistry
Surface attributes of nanoparticles are a complex idea that includes surface charge, permeability, and other variables. All this has an impact on the implementation and future of NPs for cancer chemotherapy, either actively or passively. The NPs surface is critical in influencing their numerous physical and chemical properties. Moreover, the zeta potential of NPs influences their colloidal activity, plasma protein affinity, selected NPs adherence on the cell membrane, and intracellular porosity. According to research, positively charged particles have higher uptake due to the opsonization process by plasma proteins than opposite charges [111]. Positively charged particles are widely used for antitumor delivery of drugs, and the cellular uptake of anticancer drug delivery was demonstrated using cationic particles with substantial ionic attraction with oppositely charged membrane phospholipids formed on cancer cells [111,112].
Classification of Nanoparticles
Amphiphilic polymeric Micelles
Polymeric materials like ethylene oxide or polybenzoyl Asparate, and polystyrene are being used to create polymeric micelles. As therapeutic drugs, micelles below 100 nm in size with a hydrophobic region and a hydrophilic coat are used. The hydrophilic and hydrophilic surface assists them for treating cancer since they aggregate infectious tissues. Micelles with such a tiny size are also effective for solid tumors, such as PEG- poly (D, L lactic acid), and blocked polymers like pluronic [113,114].
Liposome
These are comprised of a phospholipid bilayer and vacant cores having aqueous medium and sizes ranging from 1 to 150 nm. Liposomes are designed to transport macromolecules, which may serve as a ligand to the surface. It remains in the bloodstream for a long period and due to its hydrophobic and hydrophilic core, they are efficient against tumor cells. Positively charged liposomes are considerably more likely to be discovered in tumor tissues [115,116]. Liposomes were widely used in the treatment of cancer patients. Doxorubicin (DoxilTM), a PEGylated long circulating liposome filled with doxorubicin authorized in 1995, was the first nanomedicine for cancer cell trials. Its principal indications are early cervical cancer, leukemia, and Chronic HIV Kaposi’s sarcoma [50,117]. The treatment of Philadelphia chromosomenegative (Ph-ALL) patients with a nano-based vincristine sulfate liposome is MarqiboTM and pancreatic cancer with an Irinotecan liposome (OnivydeTM). Daunorubicin liposomes (DaunoXome®), doxorubicin liposomes, cisplatin liposomes, and others are used for treating cancer. Also, nano-liposome modification improves the functionality of the nano- drug system, leading to a more potent targeted impact. Temperature-sensitive liposomes might gradually load with gemcitabine and copper complexes in response to ultrasonic hypersensitivity to leak drugs with vascular endothelium [43,99,118-120].
Quantum Dots
Quantum dots are colloid luminescent semiconductors, formed of groups 2-4 or 3-5 in the Periodic table, with sizes ranging from 2 to 10 nm. Quantum dot compounds include cadmium, selenoid or telluride, indium phosphide, and indium arsenide. Quantum dots are significant because they have a wide surface area to engage drugs for concurrent selective distribution and in vivo studies or for tissue regeneration. Quantum dots also have long circulation periods and can illuminate for months in vivo and are highly effective tools for the detection of cancer [121-123].
Inorganic Vaccines
Inorganic NPs create tumor antigens that benefit from heatinduced or heat-reactive oxygen species (RoS). ZnO and FeO, which are used in nano vaccines, serve as basic units for targeting drugs. The ZnO surface has a high propensity for binding to different patterns. With MRI, FeO can provide imaging contrast for tracking the migration of vaccines as well as dendritic cells. Gold nanoparticles were already applied in nano vaccines, allowing for a rapid imaging-based forecast of immuno-modulating drug efficacy. One-fifth of the therapeutic dose of AuNPs is used to suppress tumorigenesis. According to the study, tumor absorption of antiPDLi coupled gold nanoparticles suppressed tumor growth [124-126].
Carbon Nanotubes
Carbon nanotubes (CNTs) are members of the fullerene family and are made up of carbon atoms grouped in rounds, spherical, or tubes. The most likely arrangement is a sequence of condensed aromatic rings coiled up into a tube shape. CNTs are classified into two types: single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs). [127,128]. Li and colleagues demonstrated that SWCNTs may associate with p-glycoprotein antibodies and be loaded with the anticancer drug doxorubicin. When combined with free doxorubicin, this compound showed 2-4 fold greater cytotoxicity against K562R leukemia cells. CNTs may be used as mediators in anticancer chemotherapy [129,130].
Dendrimer
Dendrimers are polymeric biomolecules with multiple arms radiating from the center, forming a 3D geometric design. Dendrimer and oligonucleotide-coupled dendrimer NPs are designed to enhance therapeutic activity through direct delivery to tumor tissue. Several types of dendrimers were developed for drug carriers, including polyamidoamine (PAMAM), polypropyleneimine (PPI), poly(glycerol-co-succinic acid), poly-L-lysine (PLL), melamine, triazine, poly(glycerol), poly[2,2-bis(hydroxymethyl) propionic acid], and poly(ethylene glycol) (PEG), as well as carbohydrate- and citric acid based [131].
Nanodiamonds and Nanoshells
Nanodiamonds have a truncated octahedral architecture and range in size from 2 to 8 nm. NDs have significant diamond properties like as chemical reactivity, severe durability, rigidity, and toughness. Nanodiamonds have a high potential for absorption. Because of their physiochemical properties, they are far more effective than other particles [132]. Carbon nanospheres are made up of a silica core surrounded by a thin metallic shell, typically made of gold. Such nanoshells scatter light and are effective for cancer imaging [133]. For instance, photothermal with gold nanoshells has been found to successfully ablate targeted tumors by releasing immunostimulatory molecules and triggering dendritic cell activation [134,135].
Recent Advancements in Immunotherapy
Immunotherapy is the treatment of certain cancers by generating, increasing, or inhibiting the immune reaction. Some methods of immunotherapy just influence specific cells of the immune system, whilst others affect the entire immune system. Some immunotherapeutic treatments are being used nowadays. In the late 1900s, William B. Coley, MD, now regarded as the Father of Immunotherapy, attempted to use the immune system to cure cancer. In 1891, William B. Coley, MD administered active and inactive Streptococcus pyogenes and Serratia marcescens to people with cancer who attempt to use their immunity to fight cancer [136]. Due to the lack of a defined MOA and the potential to infect cancer sufferers with deadly microbes, doctors preferred surgery and radiotherapy as the primary treatments [21,137]..
The first mAb granted FDA approval for anticancer therapy was in 1997. In 2010, the FDA validated sipuleucel-T, a tumor vaccine,\ for castration-resistant ovarian cancer. In 2011, a CTLA-4-targeting antibody was licensed for melanoma. The investigational anti- PDL1 antibody (MPDL3280A) has also yielded promising results in carcinoma, lung cancer, and bowel cancer. Antibody inhibiting PD-1 and PD-L1 are being explored against other malignancies [138,139]. Cancer therapy is meant to improve the immune system of the body against cancer by employing compounds created by the host and laboratories to stimulate the immune response. T-cell therapies, mAbs, cancer vaccines, or other types of therapies are used to treat various cancers. Adoptive cell transfer (ACT) is a method that aims to boost T-cells’ inherent strength to attack cancer [140].
Nanotechnology is not only used to kill cancerous cells, but it can
employ to detect them earlier. Nanoparticle or nanowire sensors
can detect proteins associated with particular types of tumors [23].
Nanoparticles benefits from new therapies in four categories.
A. Immunomodulators carried by NPs, like immune
pharmaceuticals, vaccines, and siRNAs, could be released slowly on
specific targets, extending the efficacy of therapy and minimizing
adverse reactions.
B. Photothermal action of NPs induces cell damage.
C. Engineered NPs may stimulate cytotoxic T and APCs,
invert macrophage polarity, and suppress Treg cells, increasing
cancer eradication.
D. Targeting tumor blood vessels and the hypoxia milieu not
only raises immunotherapeutic sensitivity but also improves the
therapeutic benefit of chemotherapy and radiation. [141].
Human papillomavirus (HPV) causes warts are mild skin cancers treated to eradicate the HPV- triggered epidermal warts by activating the immune response [142]. Tumor-associated antigen and suitable adjuvant that engages DCs and tumor-specific T cells to trigger an anti-tumor response. Some treatments were developed, despite their certain limitations because of the complexity and variety of cancers, and also TME, which reduces the potency and effectiveness of immunization [23].
In 1980, nanoscience used NPs drug loaded to optimize their accessibility, protect drugs from destruction, and prolong their half-life. Antigens, vaccines, adjuvants, mediators, and antibody aggregate in APCs due to specific physiochemical features. This influx triggers the CD8+ effector, cytotoxic T lymphocytes detect and kill tumor cells via TCR and MHC interaction. It altered the TME and awakened the immune function. The use of nanoparticles boosts the generation of cytokines, which regulate cellular and humoral response [143]. Adjuvants are substances that can activate the immune system and promote the pathogenicity of antigens, may restrict the rate of antigen disintegration, and slow their release into the lymphatic vessels, leading to sustained immune activation [144]. Polymeric nanoparticles with sizes of 50 nm were shown to target lymph vessels more successfully than bigger particles; 20- 40 nm is the ideal size for NP-based vaccinations to enter lymph nodes. As a result, adjuvants are effective for antigen presentation and cancer therapy [69,145].
Nanotherapeutics is a rapidly growing field of oncology that is being applied to overcome several constraints of drug delivery platforms. Recently, nanoparticles have been employed to boost immune function in anticancer therapy. Nanoparticles were researched in the area of drug delivery because of their capacity to properly distribute drugs to target regions, defend against endogenous enzymes, and remain in circulation for extended periods. In comparison to conventional chemotherapy, nanoparticles have significant advantages. The size of the nanoparticle must be tiny enough, as measured on a nanoscale, to pass through many physical and biological limitations. The particle charge, physical properties, surface chemistry, and form of a nanoparticle help to reduce negative side effects [16].
A nanoparticle can be spherical, tube-shaped, or rod-shaped, and many more shapes have been devised. They can be divided into three types: organic NPs, inorganic NPs, and hybrid NPs. In the pharmaceutical industry, NPs enhance therapeutic activity by offering delivery methods. But, nano-based drug carriers can shield medicines from degradation before they reach their target, as well as boost drug absorption rate into tumors, prevent drugs from interacting with normal cells, and eliminate side effects [37,38]. Moreover, NPs or nanowire sensors can identify proteins from certain types of cancer cells. The research concentrate ought to be identifying nanoparticles that function well with specific chemotherapeutic agents to enhance the drug’s anticancer activity. Studies should also be encouraged to discover and develop nanoparticle-based technologies for the detection of cancer. As a result, for medication design and exploitation, a greater knowledge of the TME and deeper exploration of the interaction between NPbased drug carriers and cancer resistance are anticipated.
Conclusion
To conclude, the substance and particle shape selection, surface chemistry, packed drug variety, and delivery of drug strategies in nanomedicine all are exceedingly variable. As a result, there is a significant amount of space for advancement in nanomedicine. Biomaterials efficacy in tumor therapy has been demonstrated during the last few decades. After that, more than 10 different types of nano-based medications were approved for the clinical treatment of tumors or other disorders. In the future, the concepts we have learned from our prior knowledge of nanoparticles will lead us in designing more effective anticancer immunotherapies, the prospect of nanomedicine looks optimistic as long as the effort to strengthen the therapeutic impact of nanoparticles proceeds alongside it.
References
- Li Z, Tan S, Li S, Shen Q, Wang K (2017) Cancer drug delivery in the nano era: An overview and perspectives. Oncology reports 38(2): 611-624.
- Patra JK, Das G, Fraceto LF, Campos EVR, Torres MDPR, et al (2018) Nano based drug delivery systems: recent developments and future prospects. Journal of nanobiotechnology 16(1): 1-33.
- Chang HI, Yeh MK (2012) Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International journal of nanomedicine 7: 49.
- Park JW (2002) Liposome-based drug delivery in breast cancer treatment. Breast Cancer Research 4(3): 1-5.
- Immordino ML, Dosio F, Cattel L (2006) Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. International journal of nanomedicine 1(3): 297.
- Kamaly N, Xiao Z, Valencia PM, Radovic Moreno AF, Farokhzad OC (2012) Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chemical Society Reviews 41(7): 2971-3010.
- Sahu T, Ratreb YK, Chauhanc S, Bhaskard LVKS, Maya P et al (2021) Naire Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. Journal of Drug Delivery Science and Technology 63: 102487.
- Couzin Frankel J (2013) Cancer immunotherapy. American Association for the Advancement of Science.
- Ahmad S, Idris RAM, Hanaffi WNW, Perumal K, Boer JC (2021) Cancer nanomedicine and immune system—Interactions and challenges. Frontiers in Nanotechnology 3: 681305.
- Chidambaram M, Manavalan R, Kathiresan K (2011) Nanotherapeutics to overcome conventional cancer chemotherapy limitations. Journal of pharmacy & pharmaceutical sciences 14(1): 67-77.
- Zhang Y, Fu J, Shi Y, Peng S, Cai Y, et al (2018) A new cancer immunotherapy via simultaneous DC‐mobilization and DC‐targeted IDO gene silencing using an immune‐stimulatory nanosystem. International Journal of Cancer 143(8): 2039-2052.
- Kapse Mistry S, Govender T, Srivastava R, Yergeri M (2014) Nanodrug delivery in reversing multidrug resistance in cancer cells. Frontiers in pharmacology 5: 159.
- Zhang C, Zhou X, Zhang H, Han X, Li B, et al (2022) Recent progress of novel nanotechnology challenging the multidrug resistance of cancer. Frontiers in Pharmacology 122.
- Kumar A, Swain CA, Shevde LA (2021) Informing the new developments and future of cancer immunotherapy. Cancer and Metastasis Reviews 40(2): 549-562.
- Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, et al (2014) Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer discovery 4(9): 998-1013.
- Swann JB, Smyth MJ (2007) Immune surveillance of tumors. The Journal of clinical investigation 117(5): 1137-1146.
- Ferro K, Peub R, Yang W, Rosenstiel P, Schulenburg H, et al (2019) Experimental evolution of immunological specificity. Proceedings of the National Academy of Sciences 116(41): 20598-20604.
- Lesterhuis WJ, Haanen JB, Punt CJ (2011) Cancer immunotherapy–revisited. Nature reviews Drug discovery 10(8): 591-600.
- Rabinovich GA, Galluzzi L, Vacchelli E, Pedro JMBS, Buqué A, et al (2014) Classification of current anticancer immunotherapies. Oncotarget 5(24): 12472-12508.
- Galluzzi L, Vacchelli E, Pedro JMBS, Buqué A, Senovilla L, et al (2014) Classification of current anticancer immunotherapies. Oncotarget 5(24): 12472.
- Naran K, Nundalall T, Chetty S, Barth S (2018) Principles of immunotherapy: implications for treatment strategies in cancer and infectious diseases. Frontiers in microbiology 9: 3158.
- Navya P, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, et al (2019) Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano convergence 6(1): 1-30.
- Sebastian R (2017) Nanomedicine-the future of cancer treatment: a review J Cancer Prev Curr Res 8(1): 204-265.
- Hurder AG, IND#: 130630 IND Sponsor: Priscilla Brastianos MD
- Chiocca E, Rabkin S (2014) Oncolytic Viruses and Their Application to Cancer Immunotherapy (vol 2, pg 295, 2014). Cancer Immunology Research 2(7): 699-699.
- Howard LK, Kaufman FJ (2015) Oncolytic viruses: a new class of immunotherapy drugs. NATURE REVIEWS. DRUG DISCOVERY 14(9): 642-662.
- Chiocca EA, Rabkin SD (2014) Oncolytic viruses and their application to cancer immunotherapy. Cancer immunology research 2(4): 295-300.
- Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nature reviews Drug discovery 14(9): 642-662.
- Cao Gd, He XB, Sun Q, Chen S, Wan K, et al (2020) The oncolytic virus in cancer diagnosis and treatment. Frontiers in Oncology 10: 1786.
- Martínez BedoyaD, Dutoit V, Migliorini D (2021) Allogeneic CAR T cells: an alternative to overcome challenges of CAR T cell therapy in glioblastoma. Frontiers in immunology 12: 640082.
- Wall D, Krueger J (2020) Chimeric antigen receptor T cell therapy comes to clinical practice. Current Oncology 27(s2): 115-123.
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH (2014) Chimeric antigen receptor therapy for cancer. Annual review of medicine 65: p. 333.
- Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W et al (2017) In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nature nanotechnology 12(8): 813-820.
- Zhang E, Xu H (2017) A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. Journal of hematology & oncology 10(1): 1-11.
- Pettinato MC (2021) Introduction to antibody-drug conjugates. Antibodies 10(4): 42.
- Ayers D, Nasti A (2012) Utilisation of nanoparticle technology in cancer chemoresistance. Journal of Drug Delivery 265691.
- Peters C, Brown S (2015) Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Bioscience reports 35(4): e00225.
- Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9(3): 34.
- King S (2013) The best selling drugs of all time; Humira joins the elite Forbes com.
- Quinteros DA, Bermúdez JM, Ravetti S, Cid A, Allemandi DA et al (2017) Therapeutic use of monoclonal antibodies: general aspects and challenges for drug delivery, in Nanostructures for Drug Delivery Elsevier 807-833.
- Scott AM, Allison JP, Wolchok JD (2012) Monoclonal antibodies in cancer therapy. Cancer immunity 12(1).
- Atherton MJ, Lichty BD (2013) Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy 5(11): 1191-1206.
- Tucci ST, Kheirolomoom A, Ingham ES, Mahakian LM, Tam SM, et al (2019) Tumor-specific delivery of gemcitabine with activatable liposomes. Journal of Controlled Release 309: 277-288.
- Martinelli C, Pucci C, Ciofani G (2019) Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL bioengineering 3(1): 011502.
- Bremer Hoffmann S, Halamoda Kenzaoui B, Borgos SE (2018) Identification of regulatory needs for nanomedicines. Journal of Interdisciplinary Nanomedicine 3(1): 4-15.
- Tinkle S, McNeil S, Mühlebach S, Bawa R, Borchard G, et al (2014) Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci 1313: 35-56.
- Dabrowska A, Thaul S (2018) How FDA approves drugs and regulates their safety and effectiveness. Washington: Congressional Research Service 1-25.
- DiMasi JA, Hansen RW, Grabowski HG (2003) The price of innovation: new estimates of drug development costs. Journal of health economics 22(2): 151-185.
- Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, et al (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature nanotechnology 11(11): 986-994.
- Yuan H, Jiang W, Roemeling CAV, Qie Y, Liu X, et al (2017) Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nature nanotechnology 12(8): 763-769.
- Smith DM, Simon JK, Baker JR (2013) Applications of nanotechnology for immunology. Nature Reviews Immunology 13(8): 592-605.
- Irvine DJ, Hanson MC, Rakhra K, Tokatlian T (2015) Synthetic nanoparticles for vaccines and immunotherapy. Chemical reviews 115(19): 11109-11146.
- Grabowski N, Hillaireau H, Vergnaud J, Tsapis N, Pallardy M et al, (2015) Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. International journal of pharmaceutics 482(1-2): 75-83.
- Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271(5256): 1734-1736.
- Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal 11(11): 3887-3895.
- Mikelez Alonso I, Aires A, Cortajarena AL (2020) Cancer nano-immunotherapy from the injection to the target: The role of protein corona. International Journal of Molecular Sciences 21(2): 519.
- Gavas S, Quazi S, Karpiński TM (2021) Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Research Letters 16(1): 1- 21.
- Xia W, Tao Z, Zhu B, Zhang W, Liu C, et al (2021) Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. International Journal of Molecular Sciences 22(17): 9118.
- LiP, Wang D, Hu J, Yang X (2022) The role of imaging in targeted delivery of nanomedicine for cancer therapy. Advanced Drug Delivery Reviews 114447.
- Dreaden EC, Austin LA, Mackey MA, El Sayed MA (2012) Size matters: gold nanoparticles in targeted cancer drug delivery. Therapeutic delivery 3(4): 457-478.
- Barua S, Mitragotri S (2014) Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano today 9(2): 223-243.
- Gigliobianco MR, Casadidio C, Censi R, Martino PD (2018) Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics 10(3): 134.
- Sandin LC, Eriksson F, Ellmark P, Loskog AS, Tötterman TH, et al (2014) Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology 3(1): e27614.
- Sutradhar KB, Khatun S, Luna IP (2013) Increasing possibilities of nanosuspension. Journal of nanotechnology 2013.
- Nadaroglu H, Güngör AA, Selvi I (2017) Synthesis of nanoparticles by green synthesis method. International Journal of Innovative Research and Reviews 1(1): 6-9.
- Yaqoob AA, Ahmad H, Parveen T, Ahmad A, Oves M et al, (2020) Recent advances in metal decorated nanomaterials and their various biological applications: a review. Frontiers in chemistry 8: 341.
- Buabeid MA, Arafa ESA, Murtaza G (2020) Emerging prospects for nanoparticle-enabled cancer immunotherapy. Journal of Immunology Research 2020.
- Akakuru O, Louis H, Oyebanji OO, Ita BI , Amos PI et al, (2018) Utility of nanomedicine for cancer treatment. J Nanomed Nanotechnol 9(1): 1-6.
- Pio R, Ajona D, Espinosa SO, Mantovani A, Lambris JD (2019) Complementing the cancer-immunity cycle. Frontiers in immunology 10: 774.
- Mi Y, Hagan CT, Vincent BG, Wang AZ (2019) Emerging nano‐/microapproaches for cancer immunotherapy. Advanced Science 6(6): 1801847.
- Nakayama M (2015) Antigen presentation by MHC-dressed cells. Frontiers in immunology 5: 672.
- Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC et al, (2020) Dendritic cell biology and its role in tumor immunotherapy. Journal of hematology & oncology 13(1): 1-18.
- Gong Y, Fan Z, Luo G, Yang C, Huang Q et al, (2019) The role of necroptosis in cancer biology and therapy. Molecular cancer 18(1): 1-17.
- Park W, Heo YJ, Han DK (2018) New opportunities for nanoparticles in cancer immunotherapy. Biomaterials research 22(1): 1-10.
- Grimaldi AM, Incoronato M, Salvatore M, Soricelli A (2017) Nanoparticle-based strategies for cancer immunotherapy and immunodiagnostics. Nanomedicine 12(19): 2349-2365.
- Finn OJ (2017) Human tumor antigens yesterday, today, and tomorrow. Cancer immunology research 5(5): 347-354.
- Buonaguro L, Tagliamonte M (2020) Selecting target antigens for cancer vaccine development. Vaccines 8(4): 615.
- Allegra A, Gioacchino M, Tonacci A, Petrarca C, Gangemi S (2021) Nanomedicine for Immunotherapy Targeting Hematological Malignancies: Current Approaches and Perspective. Nanomaterials 11(11): 2792.
- Qi S, Wang X, Chang K, Shen W, Yu G, et al (2022) The bright future of nanotechnology in lymphatic system imaging and imaging-guided surgery. Journal of Nanobiotechnology 20(1): 1-27.
- Cheng Z, Que H, Chen L, Sun Q, Wei X (2022) Nanomaterial-based Drug Delivery System targeting lymph nodes. Pharmaceutics 14(7): 1372.
- Thomas SN, Rohner NA, Edwards EE (2016) Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annual review of biomedical engineering 18: 207.
- Schudel A, Francis DM, Thomas SN (2019) Material design for lymph node drug delivery. Nature Reviews Materials 4(6): 415-428.
- Guo P, Huang J, Moses MA (2020) Cancer nanomedicines in an evolving oncology landscape. Trends in Pharmacological Sciences 41(10): 730- 742.
- Dai L, Liu J, Luo Z, Lia M, Cai K (2016) Tumor therapy: targeted drug delivery systems. Journal of Materials Chemistry B 4(42): 6758-6772.
- Nam J, Son S, Park KS, Zou W, Shea LD, et al (2019) Cancer nanomedicine for combination cancer immunotherapy. Nature Reviews Materials 4(6): 398-414.
- Jiang W, Roemeling CAV, Chen Y, Qie Y, Liu X, et al (2017) Designing nanomedicine for immuno-oncology. Nat Biomed Eng 1: 1–11.
- ShiY, Meel RVD, Chen X, Lammers T (2020) The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 10(17): 7921.
- Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF (2019) An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. Journal of Pharmacy and Pharmacology 71(8): 1185-1198.
- Misra R, Acharya S, Sahoo SK (2010) Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug discovery today, 15(19-20): 842-850.
- Tran S, Giovanni PJ, Piel B, Rai P (2017) Cancer nanomedicine: a review of recent success in drug delivery. Clinical and translational medicine 6(1): 1-21.
- Alsaggar M, Liu D (2018) Organ-based drug delivery. Journal of Drug Targeting 26(5-6): 385-397.
- Rabanel JM, Aoun V, Elkin I, Mokhtar M, Hildgen P (2012) Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Current medicinal chemistry 19(19): 3070-3102.
- Das RP, Gandhi VV, Singh BG, Kunwar A (2019) Passive and active drug targeting: role of nanocarriers in rational design of anticancer formulations. Current Pharmaceutical Design 25(28): 3034-3056.
- Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, et al (2012) Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine 7(8): 1253-1271.
- Subhan MA, Yalamarty SSK, Filipczak N, Parveen F, Torchilin VP (2021) Recent advances in tumor targeting via EPR effect for cancer treatment. Journal of personalized medicine 11(6): 571.
- Lee Y, Thompson D (2017) Stimuli‐responsive liposomes for drug delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 9(5): e1450.
- Gonzalez Avila G, Sommer B, Hernandez AAG, Ramos C, Soto EF (2022) Nanotechnology and matrix metalloproteinases in cancer diagnosis and treatment. Frontiers in Molecular Biosciences 9.
- Arslan FB, Atar KO, Calis S (2021) Antibody-mediated drug delivery. International Journal of Pharmaceutics 596: 120268.
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, et al (2016) Analysis of nanoparticle delivery to tumours. Nature reviews materials 1(5): 1-12.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, et al (2008) Nanoparticles in medicine: therapeutic applications and developments. Clinical pharmacology & therapeutics 83(5): 761-769.
- Lin PC, Lina S, Wanga PC, Sridharb R (2014) Techniques for physicochemical characterization of nanomaterials. Biotechnology advances 32(4): 711-726.
- Kumar D, Meenan BJ, Mutreja I, Raechelle ADS (2012) Controlling the size and size distribution of gold nanoparticles: a design of experiment study. International Journal of Nanoscience 11(02): 1250023.
- Zhao P, Zheng M, Yue C, Luo Z, Gong P, et al (2014) Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials 35(23): 6037-6046.
- Khan I, Saeed K, Khan I (2019) Nanoparticles: Properties, applications and toxicities. Arabian journal of chemistry 12(7): 908-931.
- Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, et al (2016) Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proceedings of the National Academy of Sciences 113(9): E1142-E1151.
- Saleh T, Shojaosadati SA (2016) Multifunctional nanoparticles for cancer immunotherapy. Human vaccines & immunotherapeutics 12(7): 1863- 1875.
- Toy R, Hayden E, Shoup C, Baskaran H, Karathanasis E (2011) The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 22(11): 115101.
- Chu M, Shao Y, Peng J, Dai X, Li H, et al (2013) Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 34(16): 4078-4088.
- Agarwal R, Singh V, Journey P, Roy K (2013) Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proceedings of the National Academy of Sciences 110(43): 17247-17252.
- Barua S, Yoo JW, Kolhar P, Wakankar A, Gokarn YR, et al (2013) Particle shape enhances specificity of antibody-displaying nanoparticles. Proceedings of the National Academy of Sciences 110(9): 3270-3275.
- Ran S, Downes A, Thorpe PE (2002) Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer research 62(21): 6132-6140.
- Yue ZG, Wei W, Lv PP, Yue H, Wang LY, et al (2011) Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 12(7): 2440-2446.
- Jhaveri AM, Torchilin VP (2014) Multifunctional polymeric micelles for delivery of drugs and siRNA. Frontiers in pharmacology 5: 77.
- Hussein HAA, Maraie NK (2021) Highlights on polymeric micelles as versatile nanocarriers for drug transporting. Al Mustansiriyah Journal of Pharmaceutical Sciences 21(2): 21-30.
- Akbarzadeh A, Maraie NK (2013) Liposome: classification, preparation, and applications. Nanoscale research letters 8(1): 1-9.
- Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. International journal of nanomedicine 10: 975.
- Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Current biology 27(14): R713-R715.
- Brien S, Schiller G, Lister J, Damon L, Goldberg S, et al (2013) High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome–negative acute lymphoblastic leukemia. Journal of clinical oncology 31(6):
- Sasaki Y, Kantarjian HM, Short NJ, Wang F, Furudate K, et al (2022) Genetic correlates in patients with Philadelphia chromosome- positive acute lymphoblastic leukemia treated with Hyper-CVAD plus dasatinib or ponatinib. Leukemia 36(5): 1253-1260.
- Passero FC, Grapsa D, Syrigos KN, Saif MW(2016) The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert review of anticancer therapy 16(7): 697-703.
- Li H, Jia C, Meng X, Li H (2019) Chemical synthesis and applications of colloidal metal phosphide nanocrystals. Frontiers in Chemistry 6: 652.
- Wagner AM, Knipe JM, Orive G, Peppas NA (2019) Quantum dots in biomedical applications. Acta biomaterialia 94: 44-63.
- Walling MA, Novak JA, Shepard JRE (2009) Quantum dots for live cell and in vivo International journal of molecular sciences 10(2): 441-491.
- Sharma P, Jang NY, Lee JW, Park BC, Kim YK, et al (2019) Application of ZnO-based nanocomposites for vaccines and cancer immunotherapy. Pharmaceutics 11(10): 493.
- Tian H, Zhang T, Qin S, Huang Z, Zhou L, et al (2022) Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. Journal of Hematology & Oncology 15(1): 1-40.
- Carabineiro SAC (2017) Applications of gold nanoparticles in nanomedicine: recent advances in vaccines. Molecules 22(5): 857.
- Saifuddin N, Raziah A, Junizah A (2013) Carbon nanotubes: a review on structure and their interaction with proteins. Journal of Chemistry 2013.
- He H, Pham Huy LA, Dramou P, Xiao D, Zuo P, et al (2013) Carbon nanotubes: applications in pharmacy and medicine. BioMed research international 2013.
- Zhang W, Zhang Z, Zhang Y (2011) The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale research letters 6(1): 1-22.
- Sharma P, Mehra NK, Jain K, Jain NK (2016) Biomedical applications of carbon nanotubes: a critical review. Current drug delivery 13(6): 796-817.
- Jabir NR, Tabrez S, Ashraf G, Shakil S, Damanhouri GA, et al (2012) Nanotechnology-based approaches in anticancer research. International journal of nanomedicine 7: 4391.
- Zhu Y, Li J, Li W, Zhang Y, Yang X, et al (2012) The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics 2(3): 302.
- Wang YC, Rhéaume E, Lesage F, Kakkar A (2018) Synthetic methodologies to gold nanoshells: an overview. Molecules 23(11): 2851.
- Wahren Herlenius M, Dörner T (2013) Immunopathogenic mechanisms of systemic autoimmune disease. The Lancet 382(9894): 819-831.
- LiuZ, Jiang W, Nam J, Moon JJ, Kim BYS (2018) Immunomodulating nanomedicine for cancer therapy. Nano letters 18(11): 6655-6659.
- McCarthy EF (2006) The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. The Iowa orthopaedic journal 26: 154-158.
- Koury J, Lucero M, Cato C, Chang L, Geiger J, et al (2018) Immunotherapies: exploiting the immune system for cancer treatment. Journal of immunology research 14:9585614.
- Herbst RS, Gordon MS, Fine GD, Sosman JA, Soria JC, et al (2013) A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. American Society of Clinical Oncology
- PowlesT, Vogelzang NJ, Fine GD, Eder JP, Braiteh FS, et al (2014) Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC). American Society of Clinical Oncology.
- Ventola CL (2017) Cancer immunotherapy, part 1: current strategies and agents. Pharmacy and therapeutics 42(6): 375-383.
- Li W, Peng A, Wu H, Quan Y, Li Y, et al (2020) Anti-cancer nanomedicines: a revolution of tumor immunotherapy. Frontiers in Immunology 11: 601497.
- Hammad NM, Abdelhadi AA, Fawzy MA, Marei A (2020) Complement component 3c and tumor necrosis factor-α systemic assessment after Candida antigen immunotherapy in cutaneous warts. Brazilian Journal of Microbiology 51(4): 1673-1681.
- Vermaelen K (2019) Vaccine strategies to improve anti-cancer cellular immune responses. Frontiers in immunology 10: 8.
- Shakya AK, Nandakumar KS (2013) Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. Journal of the Royal Society Interface 10(79): 20120536.
- Xia Y, Fan Q, Hao D, Jie W, Ma G (2015) Chitosan-based mucosal adjuvants: Sunrise on the ocean. Vaccine 33(44): 5997-6010.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.